Scientists at Duke University say they made the first time-lapse movies of the sheet-like latticework that surrounds and supports most animal tissues. A thin layer of extracellular matrix known as the basement membrane lines many surfaces of the body such as the skin, blood vessels, and urinary tract; it also surrounds muscles, fat, and peripheral nerves.
While basement membranes play key roles in development, tissue function, and human disease, visualizing them in living organisms has been difficult to do, until now. By genetically modifying C. elegans worms to create basement membrane proteins that glow under fluorescent light, the researchers say it’s possible to see for the first time how basement membranes are assembled during development, and how they change and regenerate throughout life.
This research “Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement Within the Matrix Scaffolding”, which appears in Developmental Cell, may help to pinpoint what might be going wrong in human diseases ranging from kidney disease to invasive cancer, according to the team.
“We wouldn’t be here without basement membranes,” said Duke biology professor David Sherwood, PhD, who led the research.
Basement membranes have been around for more than 600 million years, since the first multicellular animals evolved from their single-celled ancestors. They help attach cells together to form tissues, maintaining healthy skin. They’re the molecular sieves that filter blood in the kidneys, protect blood vessels, and muscles from stretching and compression, and harbor growth factors that tell cells where to go, what to become, and when to divide.
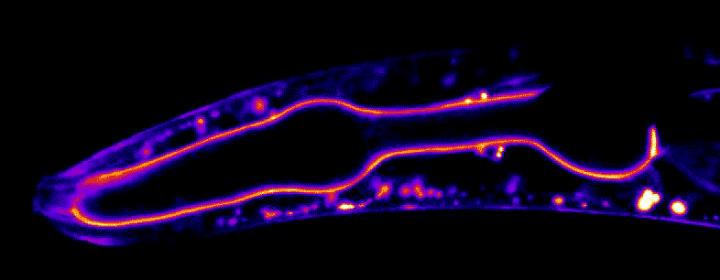
So Sherwood’s team looked at them in millimeter-long transparent worms, using CRISPR to label 29 basement membrane proteins with green glowing tags to see when and where each protein is found using time-lapse microscopy.
Getting a glimpse of these proteins in action inside a live animal offers a much more complete picture than previous experiments that looked at dissected and fixed tissues, which only provide a snapshot of proteins frozen in time, said postdoctoral fellow Eric Hastie, PhD.
“Using genome editing, we tagged 29 BM matrix components and receptors in C. elegans with mNeonGreen. Here, we report a common template that initiates BM formation, which rapidly diversifies during tissue differentiation. Through photobleaching studies, we show that BMs are not static-surprisingly, many matrix proteins move within the laminin and collagen scaffoldings,” write the investigators.
“Finally, quantitative imaging, conditional knockdown, and optical highlighting indicate that papilin, a poorly studied glycoprotein, is the most abundant component in the gonadal BM, where it facilitates type IV collagen removal during BM expansion and tissue growth. Together, this work introduces methods for holistic investigation of BM regulation and reveals that BMs are highly dynamic and capable of rapid change to support tissues.”
In some movies, the researchers tracked fluorescent proteins moving within the basement membrane lining the worm’s throat. In others, they watched the rapid remodeling of the basement membrane surrounding the worm’s gonad as it grew more than 90-fold in size.
Surprisingly, the movies show that most basement membrane proteins don’t stay put after they’re deposited. While some core components are static, the scientists were surprised to see that many proteins moved within this stable scaffolding.
“Our findings suggest basement membranes quickly change their properties to support mechanically active tissues and they may act as highways that allow growth factors to rapidly travel,” Sherwood said.
“We’ve just started getting to play with this tool kit,” noted Hastie.
But the team says their work offers a new way to study the basement membrane defects underlying tissue degeneration during aging, and diseases ranging from diabetes to muscular dystrophy.


